About
Marpé Technologies is a science based company developing innovative scanners for early detection of skin cancer.
Prototype scanners are operating at the Tel Aviv Sourasky Medical Center and the Bnei Zion Medical Center in Haifa, both leading hospitals in Israel, indicating the superior features of Marpe’s technology.

Press Releases
November 21, 2023:
Marpé Technologies and Mayo Clinic Announces Successful Installation of Marpé Technologies’ Dermatology Scanner
July 31, 2023:
Marpe Technologies Receives FDA Breakthrough Device Designation for its Revolutionary Dermatology Screening System
July 13, 2021:
Marpé Technologies Joins NVIDIA Inception
June 02, 2021:
Nortech Systems announces Cooperation and Project Funding Agreement with Marpé Technologies and the BIRD Foundation
January 19, 2021:
BIRD – Israel-U.S. Binational Industrial R&D Foundation to invest
$7.45 million in 8 new projects
December 28, 2020:
Marpe Technologies Tech-Aided Dermatology Project
The Need
Preventive Screening
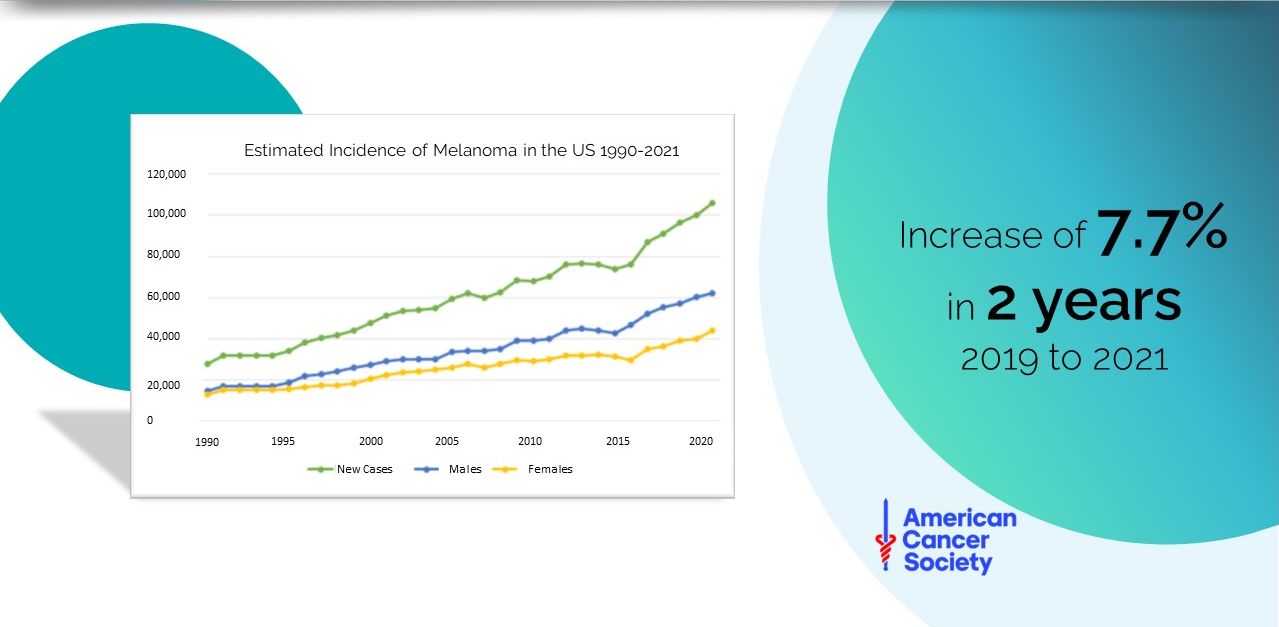
Skin diseases
- The 4th most common cause of non-fatal disease burden worldwide
- The risk of developing skin diseases – particularly cancer – increases significantly with age
.

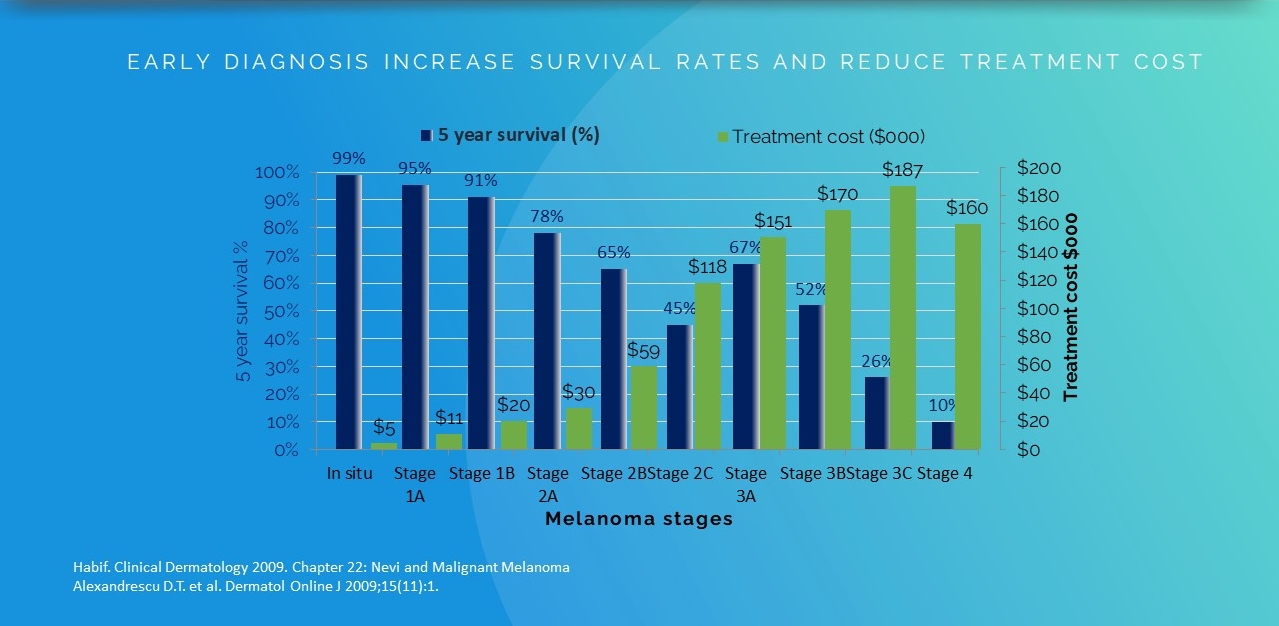
For skin cancer, early detection is vital:
- 80% of dermatologists manually perform a full-body screening of their patients
- 56% of melanomas detected in private clinics were found by a manual body scan initiated by the dermatologist
Malignant melanoma:
FACTS AND STATISTICS
Importance of
EARLY MELANOMA DETECTION
But there aren’t enough dermatologists
(use case: the US) …
The ACS recommends annual skin screening for people over 40
- The US population over 40 is 156 million
- There is a severe shortage of dermatologists (there are only 15,000 dermatologists in the US)
- The examination process is time consuming (takes over 30 minutes)
The result
%
of US population have accessibility to preventive skin screening
The dermatology sector will evolve, it’s just a matter of time… and technology
Our Solution
Marpé Technologies’ vision:
Evolving dermatology with 21st Century technology
Tech-Aided Dermatology (TAD) system that will:
- Set a new Gold Standard in dermatology
- Eliminate the overload on dermatologists
- Bring full body Remote Diagnosis & Prevention into dermatology

The new patient journey in tech-aided dermatology

AI Analysis for Dermoscopy is the Edge
- The new patient journey in TAD is based on the system’s ability to identify suspicious nevus / moles
- This is the competitive edge that enables remote diagnosis in dermatology
- Marpé Technologies offers the first viable solution: early tests indicate >98% positive identification for 1,000 scanned patients!
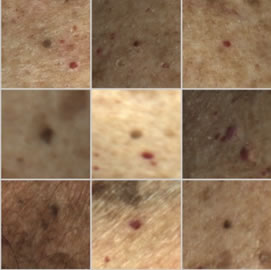
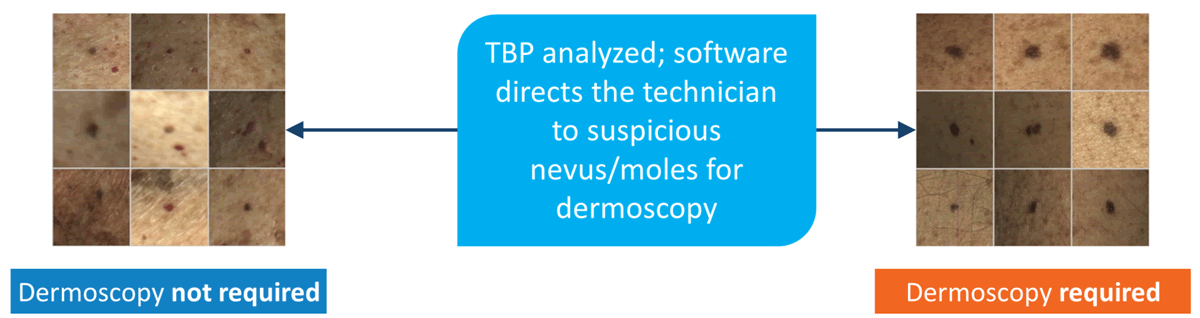
Dermoscopy not required
software directs the technician to suspicious nevus/moles for dermoscopy
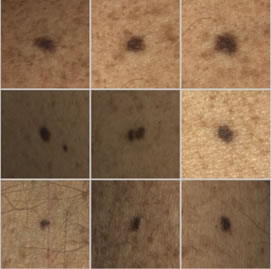
Dermoscopy required
The Product
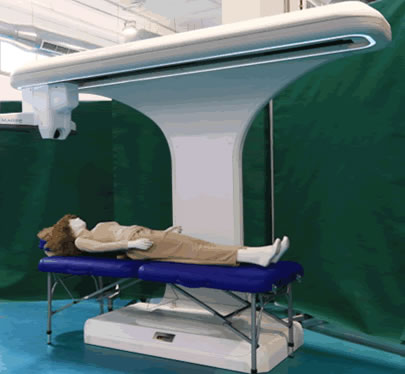
The System
- Marpé Technologies’ system is unmatched by any solution in the market
- Single image per body side – no stitching required
- Image size up to 600 MB
- Very high spatial and color resolution via German-made tri-linear sensors (RGB) line camera and built-in lighting
- Life-like navigation system operated by a technician
- Remote guidance via CAD software
Full body scan under 10 minutes
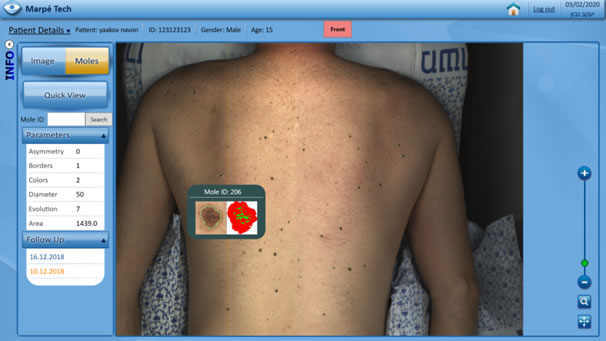
The Software
- A state-of-the-art Computer-Aided Decision (CAD) solution
- Detection of suspicious skin lesions requiring dermoscopy (nevus/moles) = over 95%
- AI-based prediction based on sequential comparison of suspicious moles
- High-end workstation and user-friendly interface for fast & precise diagnosis

A Breakthrough Software in AI
Marpé Technologies collaborates with the Israeli Institute of Technology – the Technion
The purpose: determining the software accuracy and precision in automatic detection of nevus/moles under visible light
The goal: beating 95% accuracy in detection of nevus/moles
The final accuracy of the network is 99.8% and its precision is 92.7% = overall detection rate of >95%



Collaboration







The Team

Tovi Bachar
CEO, B.Sc. Degree and M.Sc.
Over 30 years of experienced manager, Managing Director of GE Healthcare Israel.

Dr. Yaakov Navon
Founder & CTO

Dr Gila Isman Nelkenabum
CMO

Anat Kaphan
Board Member (BD)

Avichai Landau
Chief Engineer

Maya Yeheskel
CFO
Maya has over 10 years of experience in financial management and economics, extensive experience in working with the Israeli Innovation Authority incubators.

Dr Susan Alpert
Regulatory
Ph.D., M.D, Dr. Alpert serves on the Executive Committee of the Clinical Trials Transformation Initiative, one of the public/private partnerships working with FDA to streamline the development of medical products.